 Planetary Motion, Inertia And Gravity
Planetary Motion, Inertia And Gravity
People had ideas about how the universe was constructed back as far as we can see. After all, spending time looking at the heavens almost forces you to consider what it all is and how it works. And it was very easy to see that all the heavenly bodies (stars, planets, the moon) moved across the sky, from east to west, every night, without fail.
In the early 17th century, Galileo Galilei proved that heavier objects did not fall faster than lighter objects. (Heavy things strike the ground with more force than light things, but they do not fall faster.) He also proved that all things fall with a predictable acceleration. Much of this was contrary to accepted opinion.
The equipment Galileo used to make this proofs was very simple: the kind of thing you could build yourself. Here it is:

Beginning in 1609, Johannes Kepler, working from the very careful records of astronomer Tycho Brahe, figured out three laws of planetary motion. These laws described, mathematically, the moments of every planetary body that could be seen, and they still hold. With them, Kepler proved that planets orbit the sun in elipses, and not in circles as had previously been maintained.
With Kepler’s theories, people could calculate precisely how the heavenly bodies moved, and where they’d be at any moment in time. But that made a gap in human understanding even more obvious: How do they remain in these orbits?
Galileo Galilei had shown that objects in motion retain their velocity in the absence of any outside forces. We call this tendency inertia, and we often describe it by saying that objects in motion will remain in motion, unless acted upon by some other force.
Nonetheless, inertia seemed to apply only to things moving in a straight line, not things turning corners: outside forces were required to make things turn. Why, then, did everything orbit? There was clearly nothing pushing them.
Then, in 1666, Isaac Newton solved the riddle. He realized that every object was drawn to every other, through some sort of long-distance force, which he eventually called Gravity. He grasped how orbits work when considering the moon, and comparing it to things falling upon Earth. He saw that orbits were created by gravity and inertia working simultaneously, like this:
Inertia would keep our moon traveling in a straight line, out into space.
Gravity (attraction) between the Earth and the moon pulls the moon toward the much larger Earth at the same time.
And so, an orbit is created: The moon continues forward, but gravity keeps pulling it downward, bending its path into an orbit. And so the moon moves constantly around the Earth.
Newton made some measurements and came up with a clear explanation of gravity, and a simple formula by which its strength could be predicted.
Gravity works like this:
Everything in the universe attracts everything else.
The strength of attraction between any two objects is the mass of one, times the mass of the other, divided by the square root of the distance between them.
So the formula for the strength of gravity between two objects is this:
M1 x M2
D2
M1 is the mass of the first object.
M2 is the mass of the second object.
D is the distance between the two objects, and the elevated 2 means that D should be squared.
(This formula can be written with different letters, but that makes no difference to the actual things and their attraction.)
Newton went on to define our basic mechanical laws, which were necessary for all the machines of the industrial revolution to be produced intelligently and quickly. He also invented a new type of mathematics (calculus), among other things.
The Atom
It took a long time for scientists the discover and prove that the atom was the essential component of matter, and then to figure out what atoms were composed of and how they worked.
The best way to understand the importance of this is perhaps with a comment from physicist Richard Feynman:
If in some disaster all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generation, what statement would contain the most information in the fewest words? I believe it is the atomic hypothesis: that all things are made of atoms — little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another. In that one sentence, you will see, there is an enormous amount of information about the world, if just a little imagination and thinking are applied.
An atom is the smallest piece of any element, such as oxygen, carbon or gold. An atom is extremely small; a million of them, piled together, would still be too small to see. But an atom of silver, copper or anything else can’t be divided any further and still be silver or copper: it would just be disconnected particles.
In 1803 or so a chemist named John Dalton made charts, showing the weight (mass) and other characteristics of various elements. By this he created a preliminary chart of atoms. He also showed that atoms combined to make other compounds. (Like hydrogen and oxygen combining to make water.)
In 1897 a physicist named J.J. Thomson was able to produce a stream of very tiny atomic particles called electrons. (At first they were called “corpuscles” and “cathode rays.”) By shooting them through a magnetic field, and by noting where they hit detectors he had placed, he learned that the magnetic field affected their movement. In other words, he proved that these tiny particles were electrical in nature… otherwise they wouldn’t have reacted with magnetic fields.
In 1909, a physicist named Ernest Rutherford showed that an atom was not solid (as Thomson and others had thought), but had a nucleus – a heavy center piece – surrounded by empty space and nearby electrons. He and his team accomplished this by sending radioactive particles through gold foil, and noting where each ended up. Electrons that hit the nucleus bounced back, but others simply passed through the atom.
This experiment used radioactivity for the source of the particles. Radioactivity was then known, but quite incompletely. Still, it was a good source for particles, and it was understood well enough to be used this way.
As you can see in this drawing, the older model (humorously called the “plum pudding” model) had electrons embedded in the rest of the atom. In Rutherford’s new model, the electrons orbited the nucleus. We still use the same model, albeit with some variations.

Despite the efforts of Thomson and others, many scientists doubted the new atomic model. The final proof – the one that convinced almost everyone – came in 1905, when a young patent clerk named Albert Einstein published a paper on “The Brownian Motion.”
Years before, in 1827, a botanist named John Brown had noted that very small grains of pollen moved in a very random manner when placed in water. What Einstein saw, and showed mathematically, was that only very tiny particles (molecules of water) could possibly cause such movements. This convinced people that atoms and molecules (tiny combinations of atoms) were real.
What was also discovered along the way was that atoms played the central role in more or less every type of chemical reaction. In fact, it was learned that the number of electrons in an atom was at the very foundation of chemical reactions.
Every atom, it turns out, has one or more electrons orbiting its nucleus. But these electrons are not just in one orbit, but usually in two or more, each one further out that the one before it.
So, atoms have layers of orbiting electrons, zooming around at incredible speed. And because they zoom around many billions of times per second, these orbital layers are called electron shells.
What was also discovered was that the number of electrons in this outer shell (these are called valence electrons) control more or less every chemical reaction.
The number of valence electrons runs from one to eight. And here’s a drawing of five atoms linked together by sharing electrons in their outer, valence, shells:
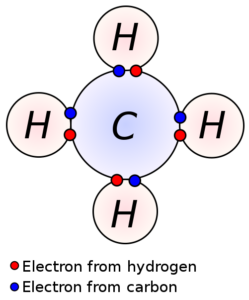
This molecule (group of joined atoms) is called methane, and it’s the “natural gas” that heats our homes and burns in our stoves. (Its chemical formula is CH4.)
An outer shell with one electron in it joins easily to other atoms, having a mainly empty shell. Lithium and sodium are atoms of this type.
An outer shell with seven electrons in it also frequently joins to other atoms, as it needs just one more electron to become a complete shell. Florine and chlorine are atoms of this type.
As people compared the various types of substances and atoms (which took many years), they began to separate them by types, as noted above for sodium, chlorine and so on. In 1865 this process had gone far enough for a Russian chemist named Dimitri Mendeleev to publish a “periodic table” of elements. Here’s a modern version of it:
We won’t go through the complexities of this table, but it lists every type of atom – every element – known to us, and it organizes them according to the number of
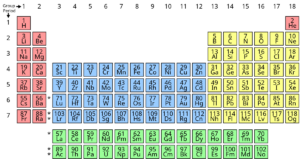
electrons, and specifically by the number of valence electrons.
From just the information we’ve included here, almost every chemical reaction can be understood: from fire for cooking, to gasoline engines, to making plastic, and to everything done in chemical laboratories.
**
Paul Rosenberg
freemansperspective.com
